Relavent
Summary[1] edit edit source
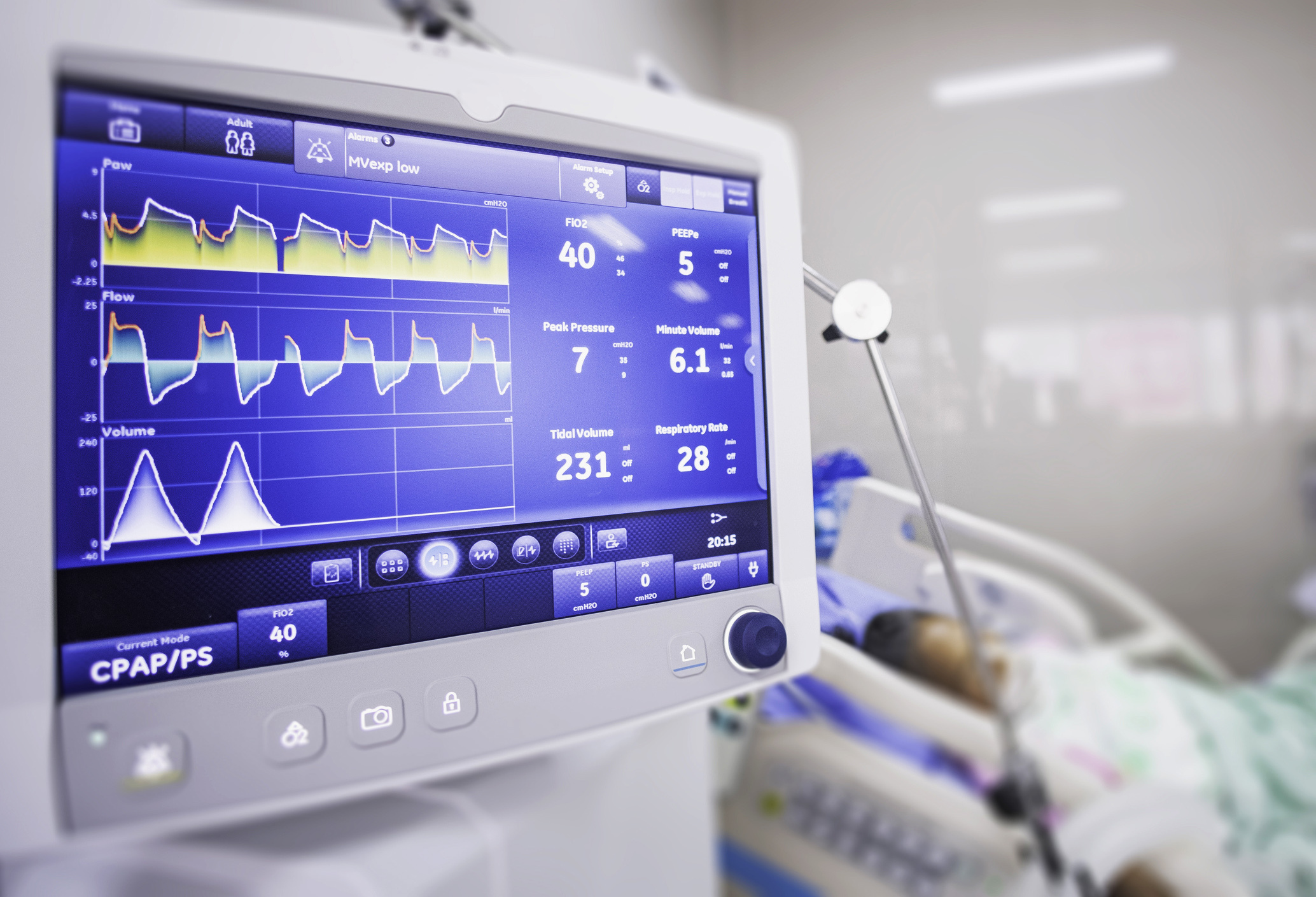
RELAVENT provides high-performance, low-cost ICU ventilators for the NEE/LMIC, ambulance and paediatric markets without relying on supply chains for specialised components used in current ventilators. RELAVENT is designed for robustness, easy maintenance, intuitive operation, and operates efficiently with low capacity gas supplies.
To become the go-to ventilation product for the large unmet need for ICU ventilators in Newly Emerging Economies (NEE) and Low-Middle Income Countries (LMIC).
Note: Working title during development was JAMVENT.
Seeking to raise £3m for a 24-month runway to reach target market, of which £2m is tentatively committed.
Investment Interview[1] edit edit source
Explain what your venture does.
RELAVENT provides high-performance, low-cost ICU ventilators for the NEE/LMIC, ambulance and paediatric markets without relying on supply chains for specialised components used in current ventilators. Its operation requires minimal electrical power. RELAVENT can be run with an oxygen concentrator or bottled gases in case of challenges in hospital gas supplies.
What stage is your business?
Imperial College Spinout. TRL 4.
Describe your advisers.
Liz Hughes (CEO): High sales growth portfolio. Commercial effectiveness specialist, accredited leadership coach. Business growth and turnaround leader. Steve Atkinson (Board Chair): Successful 30-year career in medical devices, entrepreneur, experienced fund raiser, business leader. Multiple successful exits.
Give an overview of your startup's financing history.
Grants from Imperial College COVID fund (£60k) and Royal Academy of Engineering (£20k). Departmental support. Used for prototyping, testing and submission to USA FDA for Emergency Use Authorization.
Explain the ownership structure of your company.
Imperial-based Founders: van Batenburg-Sherwood, Mathiszig-Lee, Madekurozwa, Moore, Watson, Bonneuil, Frattolin. Imperial College (IP ownership; Founder-Driven Route).
How many employees do you have?
2.
Please provide the name of a lawyer, who will represent you for the upcoming investment round.
Taylor Wessing, Ross McNaughton.
How much money are you seeking to raise in the current round?
£3m.
Do you have any existing commitments to the current round?
Family offices tentatively committed for £2m but seeking lead investor.
Explain why you are raising finance.
Complete the regulatory-compliant design process (6-9 months). Submit applications for regulatory approval (2-3 months). Clinical testing (2-3 months). Marketing. Distribution.
Please explain the history of your venture.
RELAVENT aims to provide reliable ICU-standard ventilators to low income markets where current ventilator technologies are unaffordable. The project was initiated in March 2020 in response to the COVID-19 pandemic, but the market to address diseases such as TB, malaria and influenza is much larger. Within 72 days of project initiation, we had developed a working prototype, tested it to ISO standards and submitted several hundred pages of documentation to the USA FDA for Emergency Use Authorization. We project a cost of <£10k for RELAVENT at a comfortable margin (compared to £20k-£70k for current ventilators). Note that RELAVENT provides fully invasive ventilator performance, unlike many of the "emergency use" designs that emerged in 2020.
RELAVENT is elegantly designed with a relatively small number of easily sourced components, and can be inexpensively maintained.
Please explain the longer term, future vision for the Company
Dominance of the large, unaddressed market niche in NEE/LIMC. No additional investment should be required to reach initial target markets. Entry into air/land ambulance markets in High Income Countries. CAGR projected to be 9.4% for ICU market and 10% for emergency response/ambulance markets.
Explain the core technologies and/or service propositions of your venture.
Award-winning, high performance ICU ventilator. Affordable. Not limited by specialised supply chain issues. RELAVENT provides a long-term solution for ICU ventilation, unlike other emergency use ventilation devices developed in 2020 (e.g., “bag squeezers”). RELAVENT’S design features minimal moving parts, and has a projected usage of >100 million breaths before any component would need replacing.
Does your commercial strategy rely on intellectual property assets?
Yes. Imperial-owned IP. Patent is in PCT phase. UK office action did not identify major issues.
What commercial progress have you made?
Business plan in place. Full financials available on request. Partnerships with two medical device design firms (one in the UK and one in India) are in place to complete regulatory-compliant design processes.
References and notes edit edit source